
Next Week in Public Health, September 19, 2025
We’ve been working on mapping relationships within HHS leadership (I forget if I mentioned this last week), but we still have some work to do. As with everything, we’re trying to juggle the different motives: financial, ideological, or personal.
Oh, maybe, just maybe, public help should be opposed to fascism. We gently explain why here:
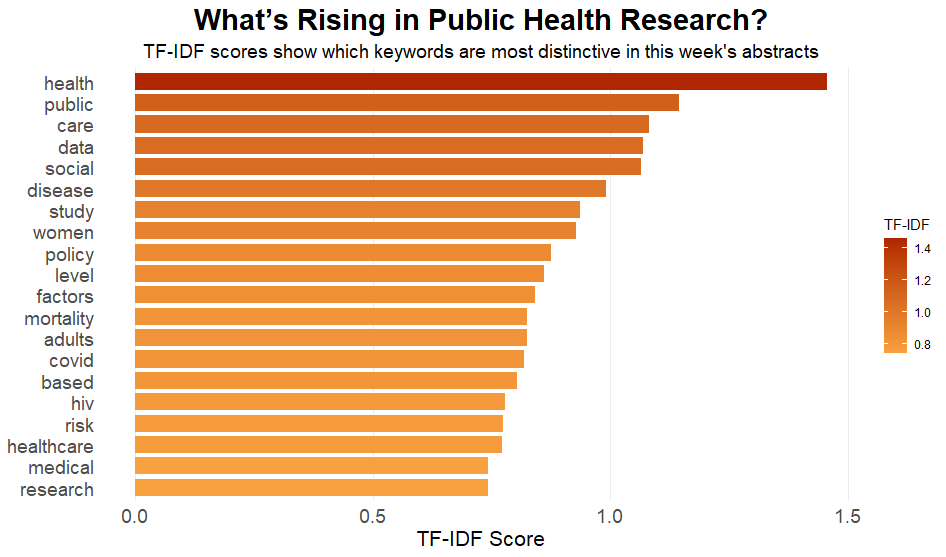
Here’s what’s in the research.
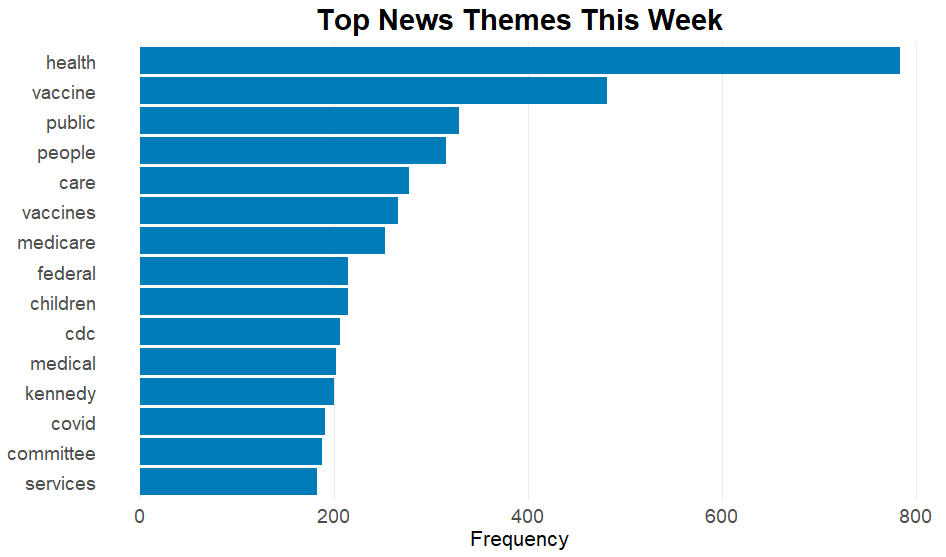
And some things in the news
Ousted CDC Director Susan Monarez testifies about RFK Jr., says she’s “very nervous” about vaccine recommendations
The Senate hearing highlighted significant tensions over the CDC’s vaccination policies, focusing on allegations against Secretary Kennedy of political interference and demands for pre-approval of vaccine recommendations without scientific review. This matters because such actions could undermine public trust in vaccination safety and efficacy, risking increased preventable diseases. The hearing also addressed CDC leadership being pressured or dismissed for resisting these demands, raising concerns about the future integrity of public health policy.
FDA in Flux — September 2025 Newsletter
The recent directives from President Trump and subsequent actions by the FDA and HHS target the accuracy and transparency of direct-to-consumer prescription drug advertisements, marking a significant shift in enforcement priorities. This initiative could disrupt established advertising strategies, affecting pharmaceutical companies and telemedicine services as it broadens the scrutiny to include digital platforms and social media influencers. Additionally, the dissolution of the DOJ’s Consumer Protection Branch and FDA’s move towards more transparency and efficiency in drug approvals, particularly for ultra-rare diseases, reflect substantial shifts in federal oversight and operational focus, potentially leading to delays and inconsistencies in enforcement and regulatory processes.
CDC’s vaccine advisory panel, with new members picked by RFK Jr., votes on measles shot
The Advisory Committee on Immunization Practices (ACIP) decided to maintain current vaccine coverage under the Vaccines for Children program, allowing coverage for both the combined MMRV vaccine and separate MMR+V vaccines for children under age 4, despite prior recommendations against the MMRV shot for this age group due to slightly increased risk of febrile seizures. This decision, amid internal confusion and external scrutiny, highlights ongoing debates over vaccine policies, underscoring the tension between scientific recommendations and political influences, impacting public trust and access to vaccines through government programs like Medicaid and Medicare. Additionally, discussions around hepatitis B vaccine recommendations indicate potential changes that could affect infant immunization practices, raising concerns about maintaining a robust public health strategy and vaccine uptake amidst political and scientific scrutiny.
AARP, Medicaid, and Changing Winds
In a recent article, an AARP Medicare Advantage (PPO) participant expressed frustration over the sudden discontinuation of their plan, requiring them to seek new coverage options by October 15 during the Annual Enrollment Period. This situation underscores broader industry changes, impacting many Medicare Advantage users who must navigate potential network changes and increased costs amid unclear policy updates due to shifts in government health policy and insurance plan realignments. This matters as it highlights the complexity and rapid changes in healthcare coverage options, urging beneficiaries to stay informed and proactively manage their health plans.
Without insurance, immigrant patients may face unregulated ‘medical deportation’
The article highlights the practice of “medical deportation,” where uninsured noncitizen patients, like Solibel Olaverria, may be returned to their home countries by U.S. hospitals due to lack of insurance and funding for long-term care. This practice often occurs in a legal and ethical gray area without standardized regulations, raising concerns about the rights of patients, the potential for non-consensual transfers, and the impact of recent U.S. policy changes that may reduce healthcare funding for immigrants. The case of Olaverria has drawn public attention and contributed to legislation in Philadelphia that seeks to protect patients by requiring written consent and reporting of medical repatriations, highlighting a growing push for transparency and patient rights in this complex intersection of healthcare and immigration.

